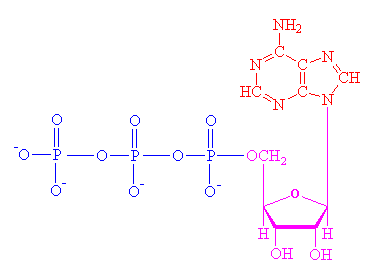
Enzymes are protein catalysts. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process, and ready to catalyze the same reaction again. Although high temperatures can increase the reaction rate, proteins are denatured at high temperatures and cannot function properly any more. In order to speed up the chemical reactions in living cells at moderate temperatures, the protein catalyst enzymes are used to lower the activation energy (Ea) barrier. Enzymes may be grouped into complexes or incorporated into membranes, specific organelle, or cytosol within the cell.
 |
| enzymes only lower Ea |
The catalyst does not affect the free energy change of a reaction or the position of equilibrium, it can only decrease the potential energy level of the transition date, therefore, allow a greater proportion of colliding reactants to reach the transition state and become products.
Substrate is the reactant that an enzyme acts on when it catalyzed a chemical reaction, and the location where the substrate binds to an enzyme is called active site. As the substrate enters the active site on the enzyme, its functional groups come close to the functional groups of a number of amino acids, then an interaction formed between these chemical groups and causes the shape of protein changes, this is known as the induced-fit model. Normally a substrate held in active site by hydrogen bonds and ionic bonds, and an enzyme-substrate complex is created. Active site can lower activation energy by acting as a template for substrate orientation, stressing the substrates and stabilizing the transition state, providing a favorable microenvironment, or participating directly in the catalytic reaction. After the reaction finished, the products are released, and the active site is available for new substrate. Different enzymes have different optimal temperatures and PH levels; the activity of enzyme is always affected by these two environmental factors. Some enzymes also require either nonprotein cofactors, such as zinc ions and manganese ions, or organic coenzymes such as NAD+, FAD+, and NADH.
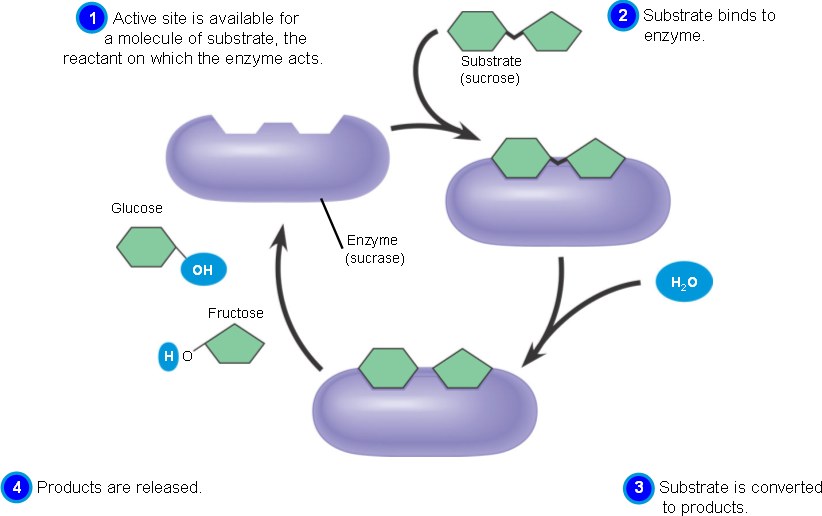
Enzyme-catalyzed reactions can be saturated, which means every enzyme is used to catalyze the reaction, and no free enzymes are available for more substrates. Therefore, a catalyzed reaction proceeds cannot increase indefinitely by increasing the concentration of the substrate.
Regulation of enzyme catalyzed reaction:
Enzyme inhibition:
Competitive inhibitors are substances that compete with the substrate for an enzyme’s active site. This process is reversible and can be overcome by increasing the concentration of the substrate. Noncompetitive inhibitors are substances that attach to a binding site on an enzyme other than the active site, causing a change in the enzyme’s shape and a loss of affinity for its substrate. Allosteric inhibitor is an example of noncompetitive inhibitor, a substance that binds to an allosteric site where is a receptor site and some distance from the active site on an enzyme, and stabilized the inactive form of the enzyme. Feedback inhibition is a method to control metabolic pathways allosterically, in which a product formed later in a sequence of reactions allosterically inhibits an enzyme that catalyzed a reaction occurring earlier in the process.
 |
| feedback inhibition |
Enzyme activation:
Enzymes can also be active by using of activators. Allosteric activator is a substance that binds to an allosteric site on an enzyme and stabilized the protein conformation that keeps all the active sites available to their substrates. Cooperativity is another type of allosteric activation, by binding one substrate molecules to active site of one subunit and locking all subunits in active conformation.

 |
| cooperativity |